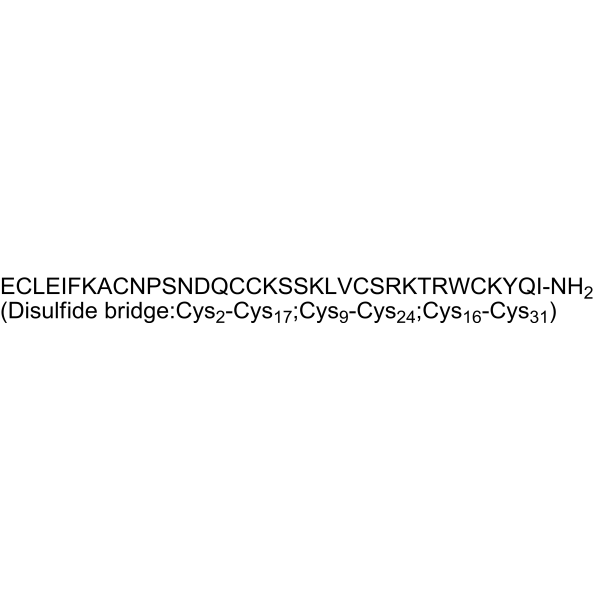
Huwentoxin IV
CAS No. 526224-73-7
Huwentoxin IV( —— )
Catalog No. M30669 CAS No. 526224-73-7
Selective NaV1.7 channel blocker. Preferentially inhibits neuronal NaV1.7, 1.2 and 1.3 (IC50 values are 26, 150 and 338 nM respectively), compared to muscle subtypes NaV1.4 and 1.5 (IC50 = >10 μM). Inhibits the channel by binding at the neurotoxin receptor site 4 in the S3-S4 linker of domain II, trapping the voltage sensor in the inward, closed configuration.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 100MG | Get Quote | Get Quote |


|
| 200MG | Get Quote | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
Biological Information
-
Product NameHuwentoxin IV
-
NoteResearch use only, not for human use.
-
Brief DescriptionSelective NaV1.7 channel blocker. Preferentially inhibits neuronal NaV1.7, 1.2 and 1.3 (IC50 values are 26, 150 and 338 nM respectively), compared to muscle subtypes NaV1.4 and 1.5 (IC50 = >10 μM). Inhibits the channel by binding at the neurotoxin receptor site 4 in the S3-S4 linker of domain II, trapping the voltage sensor in the inward, closed configuration.
-
DescriptionSelective NaV1.7 channel blocker. Preferentially inhibits neuronal NaV1.7, 1.2 and 1.3 (IC50 values are 26, 150 and 338 nM respectively), compared to muscle subtypes NaV1.4 and 1.5 (IC50 = >10 μM). Inhibits the channel by binding at the neurotoxin receptor site 4 in the S3-S4 linker of domain II, trapping the voltage sensor in the inward, closed configuration.
-
In Vitro——
-
In Vivo——
-
Synonyms——
-
PathwayMembrane Transporter/Ion Channel
-
TargetSodium Channel
-
Recptor——
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number526224-73-7
-
Formula Weight4106.79
-
Molecular FormulaC174H278N52O51S6
-
Purity>98% (HPLC)
-
Solubilitywater:1 mg/mL
-
SMILES[H]N[C@@H](CCC(O)=O)C(=O)N[C@H]1CSSC[C@@H]2NC(=O)[C@@H]3CSSC[C@H](NC(=O)[C@H](CC4=CNC5=C4C=CC=C5)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC4=CC=CC=C4)NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC1=O)[C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N3)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC2=O)C(C)C)[C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(N)=O
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Xiao et al (2008) Tarantula huwentoxin-IV inhibits neuronal sodium channels by binding to receptor site 4 and trapping the domain II voltage sensor in the closed configuration. J.Biol.Chem. 283 27300 PMID:
molnova catalog



related products
-
N-Me-aminopyrimidino...
N-Me-aminopyrimidinone9 is a sodium channel antagonist.
-
Oxethazaine
Oxethazaine is a topical anesthetic, in preventing acid-induced esophageal pain.
-
Zoniporide hydrochlo...
Zoniporide hydrochloride is a selective inhibitor of NHE-1 with IC50 of 14 nM which is >150-fold selectivity versus other NHE isoforms. Zoniporide hydrochloride shows an IC50 of 59 nM for NHE-1-dependent swelling of human platelets.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


